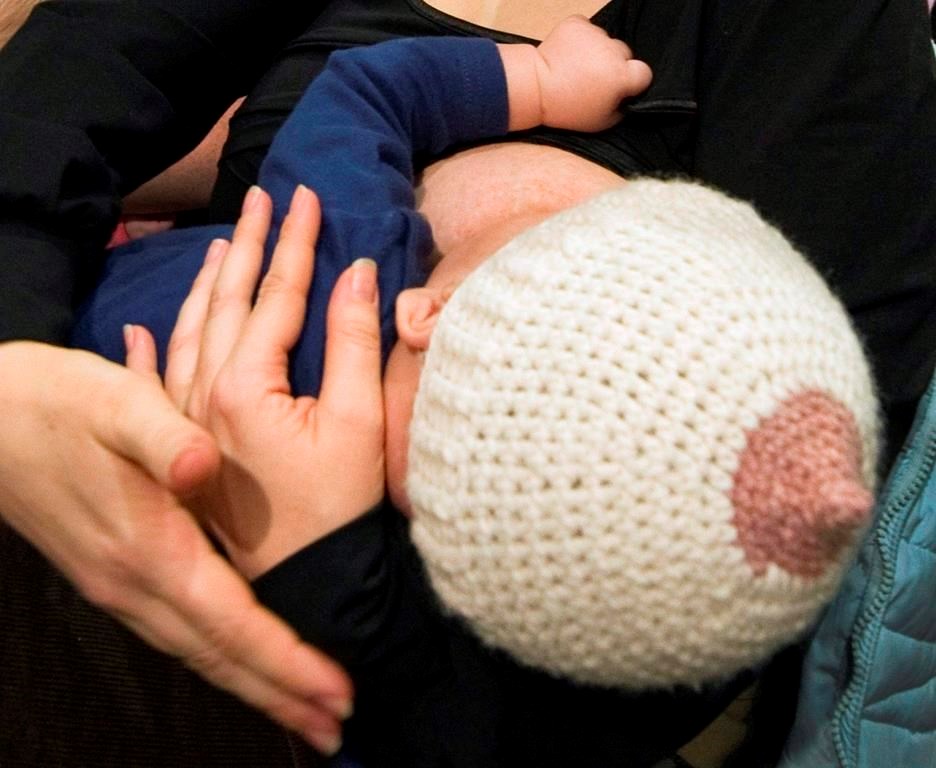
Health Canada is reviewing the safety of domperidone, a drug prescribed off-label to improve breast milk supply, amid reports that some mothers in Canada and the U.S. have had serious psychiatric symptoms when they tried to stop taking the drug.
Domperidone is approved in Canada for treating gastrointestinal symptoms but is frequently prescribed for parents facing breastfeeding challenges.
"The safety review, which started in December 2022, was prompted by domestic and foreign case reports of withdrawal symptoms after stopping or reducing the dose of domperidone used to stimulate lactation," Health Canada said in a statement on Tuesday.
"Domperidone products have not been authorized by Health Canada for use in lactation promotion."
When used to help with breastfeeding, domperidone is routinely prescribed in higher amounts than the 30 mg a day approved by Health Canada for gastrointestinal symptoms. The International Breastfeeding Centre, a well-known clinic based in Toronto, "generally" recommends breastfeeding mothers start with 90 mg a day, according to its website.
In early December, CBC News reported cases of women experiencing serious symptoms including severe anxiety, depression, intrusive disturbing thoughts and insomnia while trying to stop taking the drug.
Case studies have also documented those effects, including a research paper published in December by the InfantRisk Center at Texas Tech University Health Sciences Center.
Although he's not aware of any clinical trials examining possible psychiatric effects of domperidone among breastfeeding mothers, it's important to pay attention to the "signal" these case studies are sending, said Dr. Jonathan Zipursky, a specialist in clinical pharmacology and toxicology at Sunnybrook Health Sciences Centre in Toronto.
"This is an important potential adverse drug event that needs to be studied in a systematic way," Zipursky said.
Health Canada said the safety review "was prompted by domestic and foreign case reports of withdrawal symptoms after stopping or reducing the dose of domperidone used to stimulate lactation."
"Should new safety risks be confirmed, Health Canada will take appropriate action and continue to keep Canadians informed," it said.
The International Breastfeeding Centre says on its website that women who want to stop taking domperidone "should wean off it gradually."
Janet Currie, a research collaborator with UBC's school of nursing, has studied domperidone use among breastfeeding women and has spoken with several who struggled with psychiatric symptoms.
Currie welcomed the Health Canada safety review, but said it is "long overdue."
She worries that breastfeeding women, doctors and midwives may not have guidance on what to do while the review takes place.
"My concern is how long this will take and what will happen to mothers who are considering taking the drug, to prescribers and to those trying to reduce it in the meantime," she said in an email on Tuesday.
The FDA has banned the drug in the U.S. because of concerns about potential cardiac side effects.
"The risks of cardiac arrhythmias, cardiac arrest and sudden death outweigh any potential benefit of the unapproved use of domperidone in healthy lactating women," the agency said in a statement to The Canadian Press earlier in January.
Health Canada also warns of potential cardiac side effects on its information page about domperidone.
This report by The Canadian Press was first published Jan. 31, 2023.
Canadian Press health coverage receives support through a partnership with the Canadian Medical Association. CP is solely responsible for this content.
Nicole Ireland, The Canadian Press